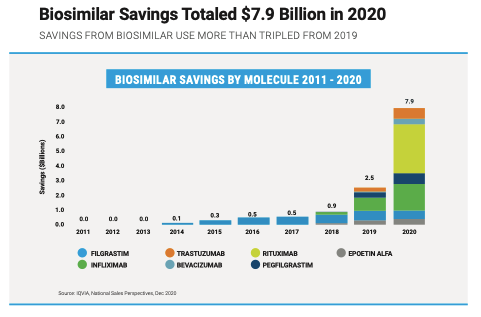
Two molecules demonstrate the great savings potential of biosimilars: infliximab and filgrastim. Multiple biosimilars have entered the market for these molecules, increasing competition and generating savings across multiple treatment areas.
When biosimilars enter the market, they are consistently launching at prices lower than that of their biologic counterpart. No biosimilar has left the market once launched, and this consistent competition and lower launch pricing has brought down the average sales price of reference biologics. This provides savings not only to insurers, employers and taxpayers, but also to patients who benefit from lower out-of-pocket costs. Collectively, the introduction and availability of biosimilars means that patient access is improved and costs are lowered.
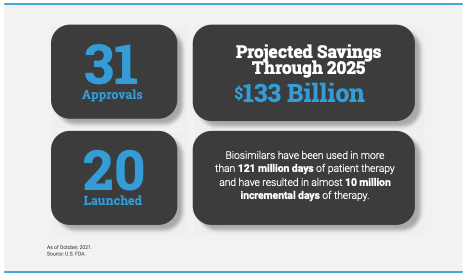
Since the approval of the first biosimilar in 2015, biosimilars have been used in more than 121 million days of patient therapy. This analysis, which was completed by IQVIA on behalf of AAM and shared by the Biosimilars Council, also found the availability of biosimilars have resulted in almost 10 million additional days of therapy.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 18 percent of total drug spending.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available at www.biosimilarscouncil.org.