Resources
Home / Resources
Providing the best advocacy resources for biosimilars.
Whether you are a patient, caregiver, medical professional, payer, policymaker, or you just want to know more about biosimilars, our resources will teach you everything you need to know. Browse through our list below to learn more, and feel free to contact us.
The Biosimilars
Patient Resource Center
The Biosimilars Patient Resource Center is a convenient site for answers to your pressing questions about biosimilar medicines.
Whether you are a current or potential biosimilar or biologics patient, a caregiver, a medical professional, a policymaker, or if you just want to know more about biosimilars, the Biosimilars Patient Resource Center has information you can use.
The Biosimilars Handbook
The Biosimilars Handbook is the premier resource for biosimilar education.
Choose from one of four different tracks below – or check out our additional suggested resources – to learn how biosimilars and interchangeable biologic products can decrease costs and increase access to lifesaving medicines. we’re advocating for right now, and make your voice heard to get these policies across the finish line.
For Patients and Caregivers
FDA – Biosimilars: What Patients Need to Know
Patients Increasingly Rely on Oncology Biosimilar Medicines
Biosimilar Medicines Equal More Accessible Care for America’s Patients
For Medical Professionals
AAM Annual Savings Report – Biosimilars and Bending the Cost Curve
Increasing Provider Reimbursement for Biosimilars Will Lead to Greater Adoption
FDA Biosimilars Approvals
For Payers
AAM Annual Savings Report – Biosimilars and Bending the Cost Curve
Increasing Provider Reimbursement for Biosimilars Will Lead to Greater Adoption
New Analyses Point to Opportunities to Increase Savings from Biosimilar Adoption
For Policymakers
The U.S. Generic & Biosimilar Medicines Savings Report
Increase Patient Access to Lower-Cost Biosimilar Medicines
Top Biosimilar Resources

Biosimilars 101
Biosimilars are a safe, effective, and more affordable option for many patients who rely on biologic treatments.

FDA Biosimilars Approvals
Find an up-to-date list of FDA-approved biosimilars and launches.
2023 U.S. Generic and Biosimilar Medicines Savings Report
This annual report is produced by the Institute for Human Data Science (IQVIA), the standard-bearer for measuring data, pharmaceutical use and spending in the United States.
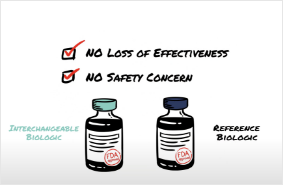
Interchangeability 101 – Biosimilars and Interchangeable Biologics
This white board video from the Biosimilars Council walks through the basics of interchangeability and why biosimilars and interchangeable biologics are more than acceptable replacements for brand biologics.
Other Resources
Filter by Keyword
More results...
- All
- Backgrounder
- Biosimilars Bulletin
- Blog
- Council Resource
- Event
- Handbook
- Infographic
- Issue Brief
- Member Spotlight
- Partner Resource
- Policy Recommendation
- Press Release
- Report
- Streamlining
- Video
- Whitepapers



Biosimilar Advocates – despite a presidential election and short Congressional calendar, the Biosimilars Council continues to advance awareness and policies… Read more »

Last week’s Festival of Biologics in San Diego came during a critical time for our industry when unfolding challenges and… Read more »

As policymakers continue to discuss ways to lower the cost of medicines for patients, the role of pharmacy benefit managers… Read more »
Last year’s launch of the first biosimilar of Humira (adalimumab) signified a major opportunity to increase patient access to, and… Read more »

As we approach the end of the year, we wanted to share a few highlights. This was an active year… Read more »


Since 2015, the U.S. Food and Drug Administration (FDA) has approved 42 total biosimilars, delivering safe and effective care for… Read more »


Biosimilars allies – as everyone returns from the summer, September marks the beginning of a busy fall for the Biosimilars… Read more »



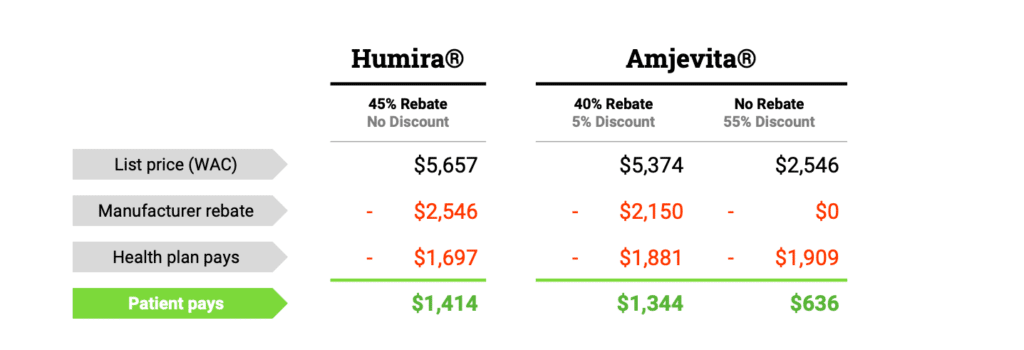




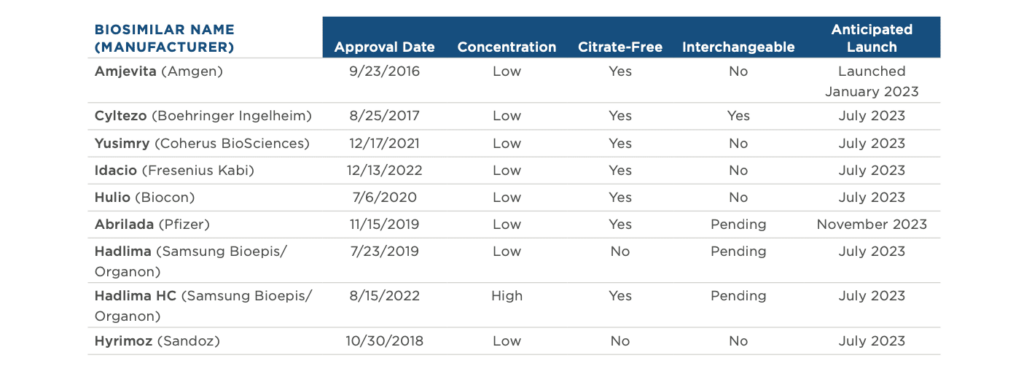

















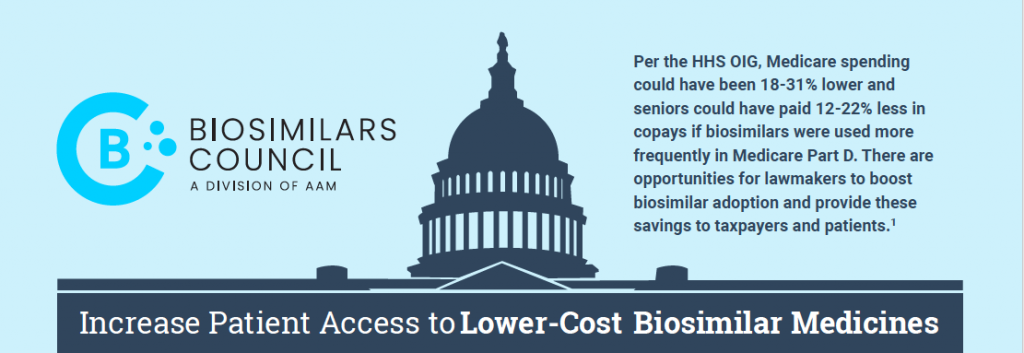


























Biosimilar competition has the potential to provide greater access to important treatments for America’s patients and cost savings for the… Read more »













































When it comes to ensuring medicines — including biosimilars — are safe and effective for patients, the FDA remains the… Read more »



Dr. Sameer Awsare, Internal Medicine Physician and Associate Executive Director at Kaiser Permanente Medical Group, sat down with the Biosimilars… Read more »

Dr. Sameer Awsare, Internal Medicine Physician and Associate Executive Director at Kaiser Permanente Medical Group, sat down with the Biosimilars… Read more »


Dr. Sameer Awsare, Internal Medicine Physician and Associate Executive Director at Kaiser Permanente Medical Group, sat down with the Biosimilars… Read more »

Dr. Sameer Awsare, Internal Medicine Physician and Associate Executive Director at Kaiser Permanente Medical Group, sat down with the Biosimilars… Read more »





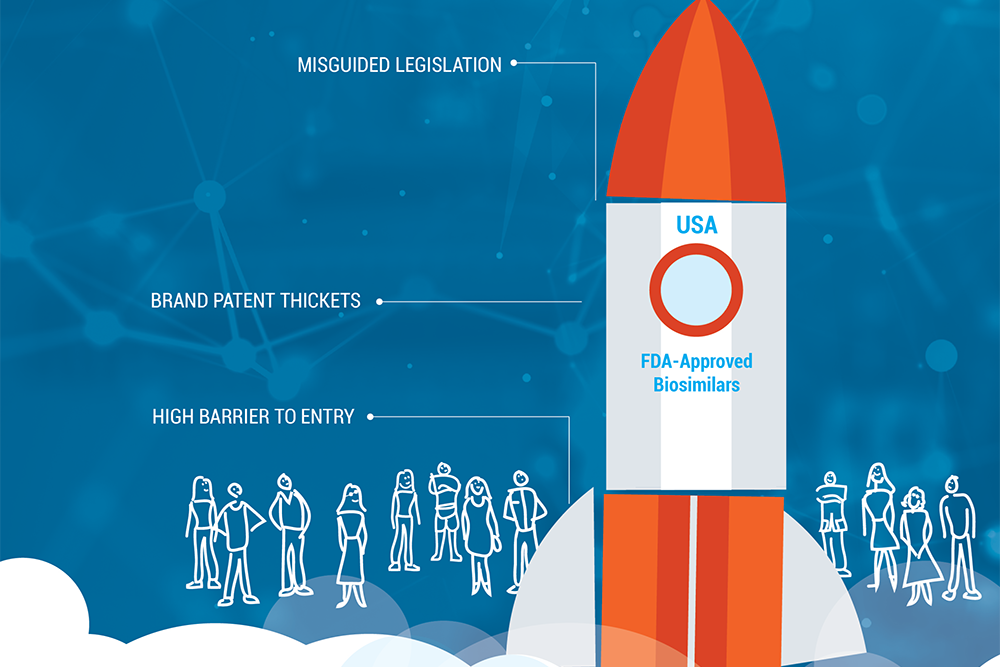
A recent series in Health Affairs questioned whether competition from biosimilar medicines is a viable path to providing relief from… Read more »
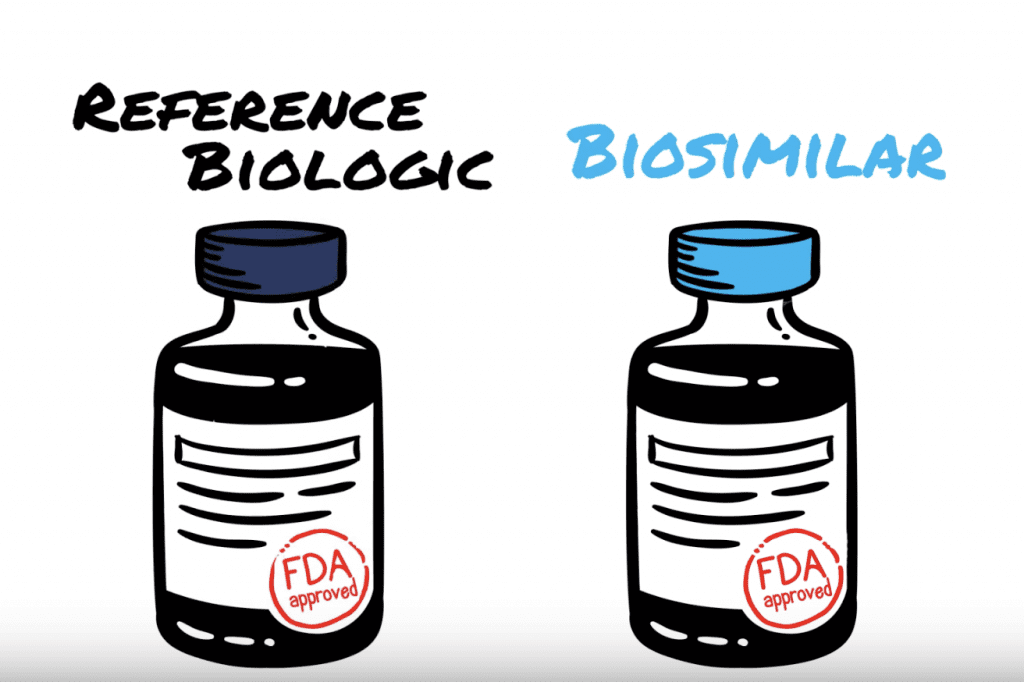






































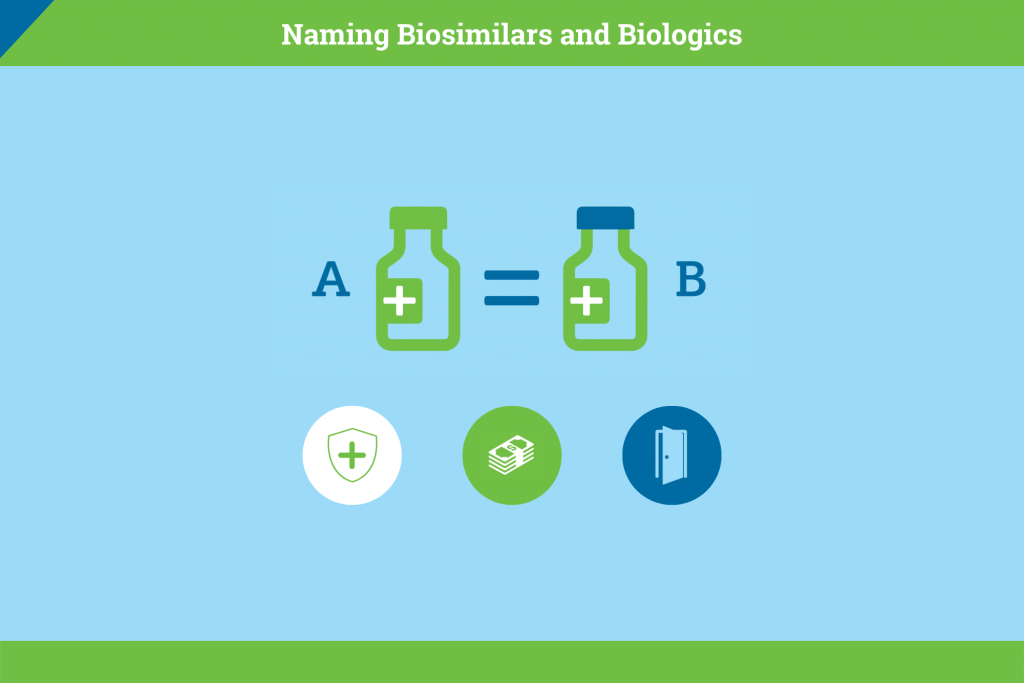
The Naming of Biosimilar Medicines Worldwide Should Promote Patient Safety and Prescriber Confidence




Biosimilars hold great promise for patients seeking access to more affordable versions of critical medicines, but also for a health care system facing unsustainable, increasing costs. One of the most immediate challenges to biosimilars uptake in the United State sis the lack of […]



In the fast-paced world of health care, the role of the patient continues to evolve from passive to active. One of the most pressing issues patients face is the cost of their medications, which can serve as a significant barrier to access, especially for expensive brand-name biologic medicines[…]


On June 7th, The Atlantic presented “The Next Drugs: The Future for Biosimilars – An Atlantic Policy Update on Biosimilars” at the Newseum in Washington, D.C. The third in a series of Atlantic biosimilars briefings underwritten by The Biosimilars Council […]

Biosimilars not only have the unique ability to provide patients with much-needed access to lower-cost complex therapies, but also offer the potential for billions in patient, insurer and health system savings. But there are barriers […]


Working in coordination with The Atlantic, an award-winning publication that covers politics, business, and technology, The Biosimilars Council will continue its efforts to educate consumers about biosimilars by bringing together key stakeholders to debate the state and future of biosimilars […]

The country has undergone a great deal of change from December to today – but one thing that has not changed is that Americans want action to bring down the price of prescription drugs. A recent Kaiser Health Tracking Poll showed that over 60% of Americans believe lowering the cost of […]

On March 15th, The Atlantic presented “The Next Drugs: The Future for Biosimilars – An Atlantic Policy Briefing” at the Newseum in Washington, D.C. Underwritten by The Biosimilars Council, more than 100 influencers from across the […]

Underwritten by The Biosimilars Council The Atlantic gathered key stakeholders to examine the emerging world of biosimilars and their regulatory framework in the United States. How does the Food and Drug Administration implement[…]

Stay Informed About The Latest Issues.
Subscribe to the monthly Biosimilars Bulletin.