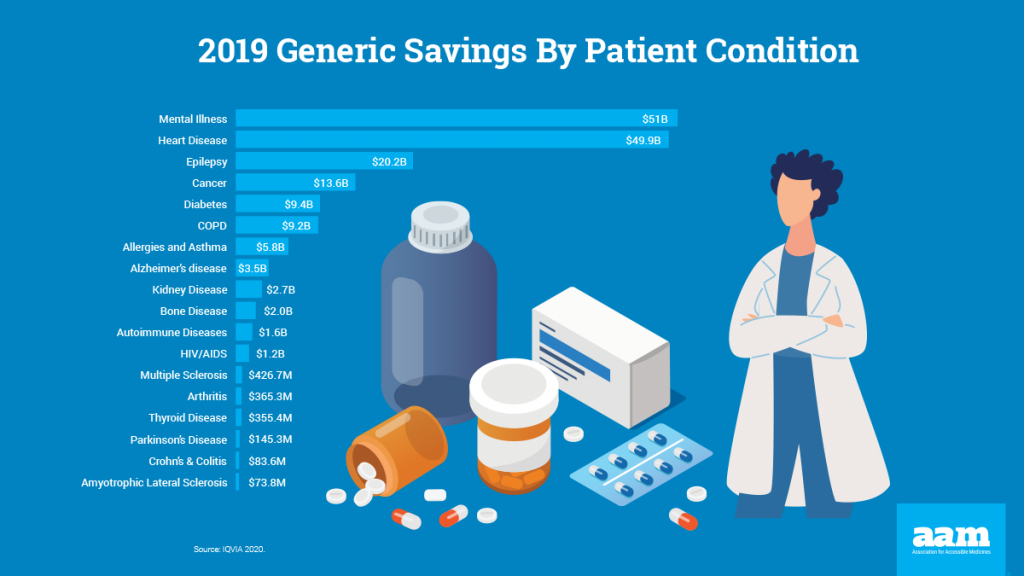
There are approximately 1.7 million new cases of cancer in the United States each year. Patients rely on FDA-approved medications to treat cancer and cancer-related symptoms. It is essential that patients can access these medicines through the pandemic and beyond. Generics and biosimilars can offer much-needed treatments with substantial cost savings.
Biosimilars Can Help Cancer Patients
Just as generics offer significant savings over brand-name medicines, biosimilars — FDA-approved alternatives to brand-name biologics — hold the promise of savings and access to cutting-edge biologic treatments. A recent ACS CAN report confirmed that patients and health care payers can save money when patients use a generic or biosimilar alternative to a branded drug. One breast cancer patient had nearly 30% savings by using a biosimilar instead of the brand-name biologic.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.