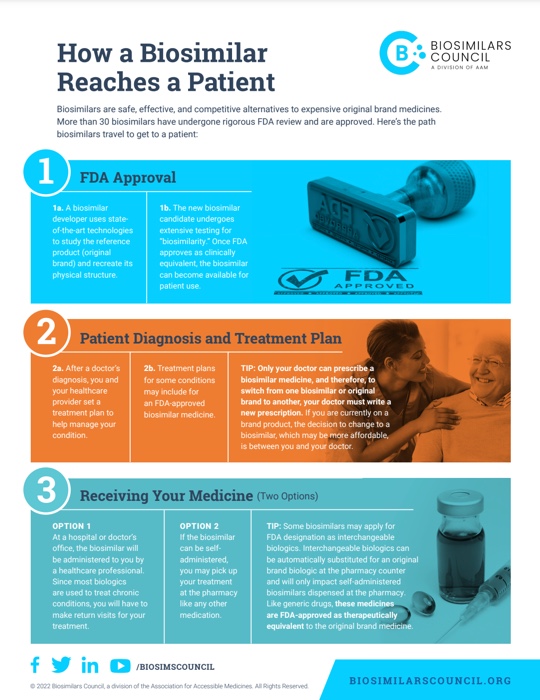
Biosimilars are safe, effective, and competitive alternatives to expensive brand-name biologics that are used to treat serious chronic diseases, like cancer, arthritis, and Crohn’s disease. Since the first one launched in 2015, biosimilars have generated billions of dollars in savings and helped patients live healthy, happy lives. So how does a biosimilar get from the lab to the patient?
This infographic lays out the process for how biosimilars are developed, approved, and prescribed, so that you can make more informed decisions.
Want to dig deeper? Visit our Biosimilars Patient Resource Center for more helpful infographics, FDA resources, patient testimonials and more.