Common sense suggests that if a lower cost product is on the market, then consumers should be able to choose that product to save money. Yet the pharmaceutical market is no ordinary market. Due to perverse incentives, many patients are denied access to lower-cost biosimilars and locked into paying high costs for brand-name medicines.
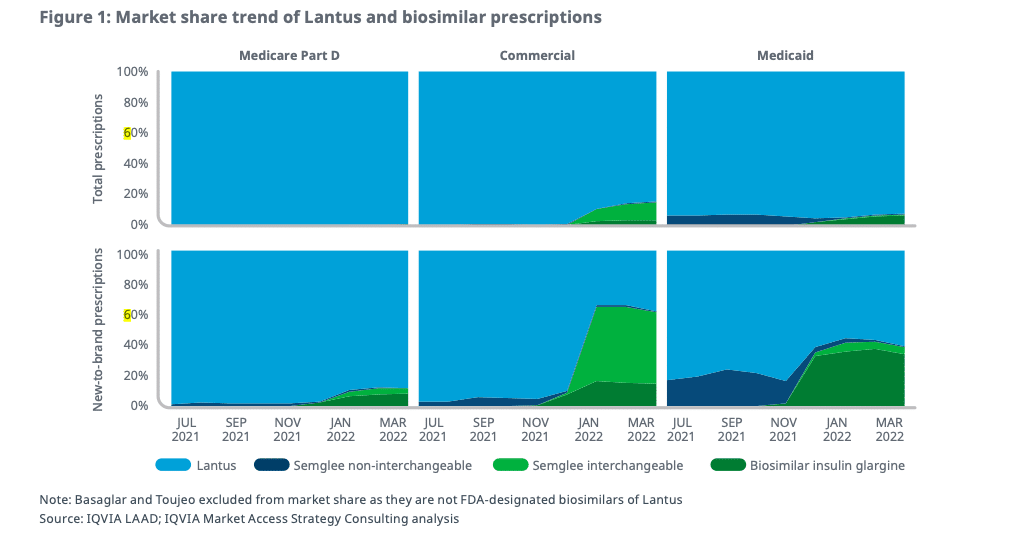
Under the current system, health plans and PBMs take advantage of brand manufacturer price concessions, such as rebates, in exchange for guaranteeing preferred placement on, as well as for excluding a lower-priced biosimilar from, a formulary. These dynamics are clearly illustrated by the limited adoption of lower cost biosimilar insulin. As data from IQVIA demonstrates, many health plans have continued to prefer and drive adoption of a higher cost brand insulin despite the existence of a biosimilar with a 65% discount. Overall, plans are choosing the products that bring in more revenue in rebate dollars but also impose higher costs on patients.
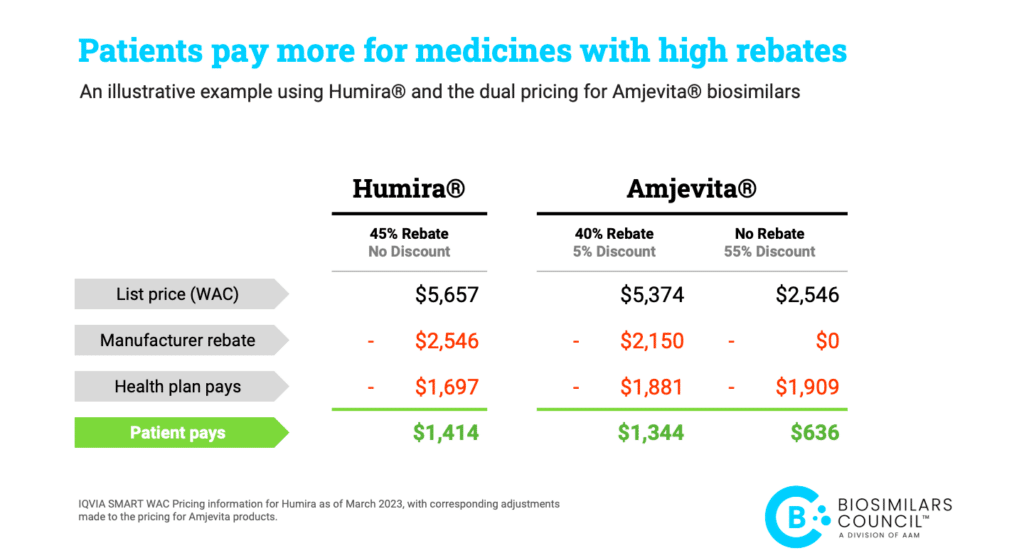
Recently, Amjevita, a biosimilar to Humira, came to the market with two price points: either a 5% discount off of the list price of Humira or a 55% discount off of list price. But early reports indicate slow plan adoption of the product overall, limited adoption of the lower priced option, and (in the instances where formularies include the biosimilar) placement at-best on parity with the brand. This means that many patients will not have access to or benefit from the biosimilar that is priced at more than half off the brand.
The result? Patients will continue to face higher costs despite the availability of a lower priced option.
A closer look at the pricing differences between Humira and Amjevita shows how patients benefit from medicines with a lower list price, rather than a higher rebate.*
As the example above illustrates, it is common for plans to save money on drugs with a high list price and a high rebate, even if patients pay more. As part of their insurance design, many patients pay co-insurance out-of-pocket for their medications. When a plan chooses a product with a higher list price over a product with a lower list price, a patient on the hook for co-insurance linked to list price pays more with every dollar of increase in the list price. In short, the plan saves money from the rebate while many patients pay more because of the higher list price.
Patients may pay less than half the cost share for the lower list price product than a higher rebate version. However, as we’ve seen with Semglee, formulary decisions are made based on what benefits the plan and the PBM, not the patient.
This demonstrates once again how perverse incentives and market distortions contribute to high drug prices for patients and the health care system. By tipping the scales in favor of offering high-cost, high-rebate treatments over lower cost alternatives, PBMs and health plans are padding their own profits while patients struggle to afford life-saving treatments.
Fortunately, policy makers can take steps to ensure formulary decisions are made with patients’ best interests and financial well-being in mind. Options include requiring plans and PBMs to pass rebates on to patients at the pharmacy counter or ensuring that Medicare formularies offer preferred coverage of new generics and biosimilars when they are lower cost.
The Inflation Reduction Act took an important first step towards encouraging plans to prefer lower cost products, but more needs to be done. Without further action, the upside-down economics favoring high-cost drugs with high rebates will continue to block patient access to new, lower-priced generics and biosimilars.
Aligning reimbursement incentives towards a more straightforward and beneficial model for competition will ensure that patients not plans save the most from new lower-priced generics and biosimilars.
*The press release for the launch of Amjevita and wholesale acquisition cost (WAC) for Humira provide topline figures and basic assumptions needed to illustrate how a patient’s copayment will differ among the products.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.