Executive Summary
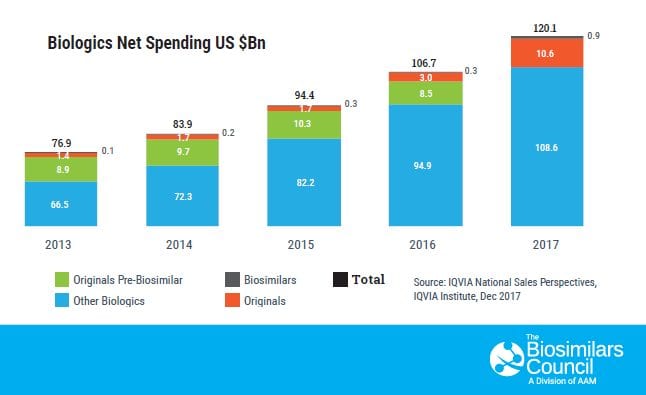
As America’s patients seek access to more-affordable versions of biologic medicines to treat their complex and chronic diseases, biosimilars offer a much-needed alternative. Today, just 2 percent of the U.S. patient population uses biologics, yet they account for 26 percent of national prescription drug spending, a record $453 billion in 2017.[1] Utilization of these innovative and very costly medicines is expected to grow exponentially in the coming years. But thanks to new biologic medicines known as biosimilars, these advanced treatments are poised to become available at a lower cost to millions of patients in the United States.
Whether the promise of biosimilars will be fulfilled for patients is largely dependent on a regulatory, legislative and market landscape for biosimilars that encourages competition and biosimilar development so that America’s patients can benefit from these life-saving therapies.
Data from Europe indicate what biosimilars mean for patients. Access to both biosimilars and biologics has increased by as much as 100 percent in Europe as the result of biosimilars availability.[2] In the U.S., biosimilars will provide greater access to biologic medicines for an additional 1.2 million patients over the next 10 years, according to a recent study.[3]
However, the U.S. trails Europe – with only 10 approved biosimilar medicines, compared to more than 40 in Europe. Only three of the FDA-approved biosimilars are available to patients in the United States.[4]
The biosimilar medicines development process requires substantial financial investment, on average estimated to cost between $100 and $300 million per product.[5] FDA Commissioner Gottlieb recently highlighted the importance of fostering the development of a strong biosimilars market, saying: “The public health benefits of a robust, competitive market for biosimilars are impossible for us to ignore. Strong market incentives are critical to future biosimilar development in the same way these incentives are key for the development of innovator drugs and biologics.”[6]
As the paper discusses, biosimilar manufacturers face regulatory uncertainty and anti-competitive market access tactics designed to prevent competition from lower-cost products. Only through dedicated policymaker attention and unbiased safety and efficacy information for patients and providers will the full promise of lower-cost, life-saving biosimilar medicines be fulfilled for patients and the U.S. health care system.
Download The Executive Summary
Sources
[1] IQVIA Institute for Human Data Science: “Medicine Use and Spending in the U.S.: A Review of 2017 and Outlook to 2022.” (April 2018)
[2] IMS Institute for Healthcare Informatics, Delivering on the Potential of Biosimilar Medicines: The Role of Functioning Competitive Markets, (March 2016). Available at: https://bit.ly/2eTu1NP.
[3] Avalere Health and The Biosimilars Council, Biosimilars in the United States: Providing More Patients Greater Access to Lifesaving Medicines. (2017)
[4] As of May 2018
[5] CNBC Interview of FDA Commissioner Gottlieb, March 28, 2018. Accessed: April 30, 2018. Available at: https://cnb.cx/2pNVVlh.
[6] FDA Commissioner Gottlieb Speech: “Capturing the Benefits of Competition for Patients,” March 7, 2018. Available at: https://bit.ly/2FuFxjN. Accessed April 30, 2018.