Each month, the Biosimilars Council sends a newsletter with the latest updates in the biosimilars industry including policy movement, educational resources and upcoming events. To stay current on biosimilars, sign-up to receive these monthly updates on the form to the right.
Biosimilars Bulletin | September 2020
News and Updates
New AAM Chief Sets Out Priorities
Biggest Influencers in Biosimilars in Q2 2020: The Top Individuals and Organizations to Follow
Sandoz President: Managing the Biologics, Biosimilars Supply Chain through COVID-19
An Update on 2020 U.S. Biosimilars Regulation and Litigation
Biosimilar Developers and the Battle Against COVID-19
Framing the Switch to Biosimilars for Patients and Providers
New Insulin and Biosimilar Regulation
Featured Resources
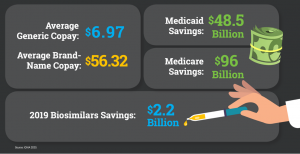
Securing Our Access & Savings

Global Roadmap for Tailored Clinical Biosimilar Development: Instrumental for Sustainable Access to Biologics
Infographic: Patients Have an Alternative to Expensive Biologic Medicines
Patients have an alternative to expensive biologic medicines. This new infographic highlights what patients need to know about biosimilars and how to increase access to these safe treatment options.

Infographic: Biosimilars Benefit Everyone
Biosimilars benefit everyone. This new infographic shows how we all — doctors, payers, and patients — can benefit from biosimilars.
Biosimilars in the Pharmacy Benefit Action Brief
This new resource from the National Alliance of Healthcare Purchaser Coalitions provides action steps for employers to drive biosimilar use and transparency.
Upcoming Events
GRx+Biosims Virtual Conference
November 9-11, 2020
Sign up now for the GRx+Biosims virtual conference.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 23 percent of total drug spending. Additional information is available at www.accessiblemeds.org.
