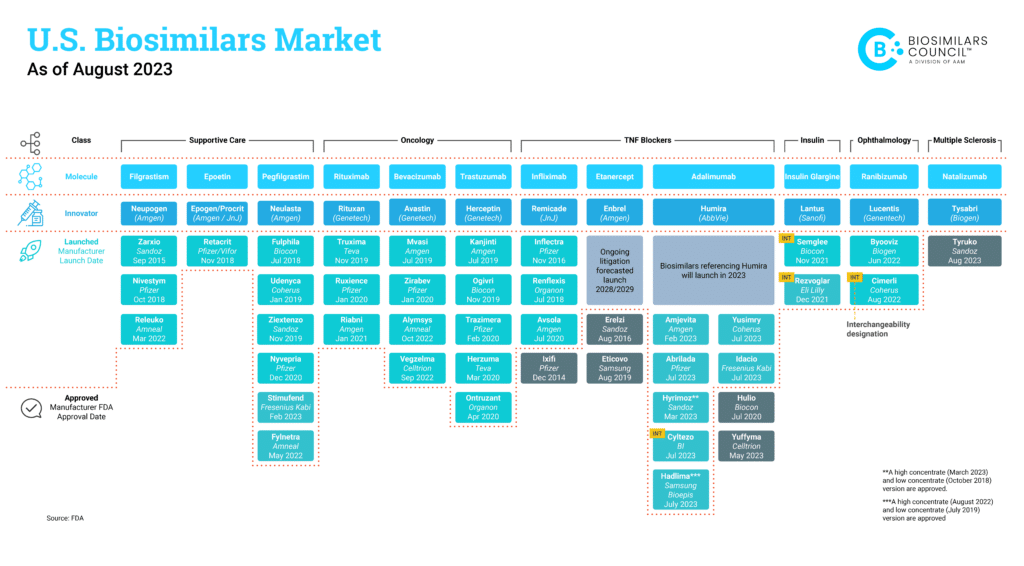
Scroll down to find an updated list of all FDA-approved biosimilars. This list includes information on when biosimilars were approved, when they became available to patients and which biologic they reference.
The market for FDA-approved biosimilars is expanding, as they now approach nearly 30% of the overall biologics market. The average sales price for biosimilars is on average 50% less than the reference brand biologic price. Further, competition from biosimilars has reduced the average sales price of their corresponding reference biologic by an average of 25%
For more information about how biosimilars benefit patients, visit our resources page or the FDA website.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.