AAM Resources
The Biosimilars Council is part of the Association for Accessible Medicines (AAM), which seeks to improve access to safe, quality, and effective medicines across the United States.
As part of that mission, AAM maintains a carefully curated and continually updated resource library with information on both generics and biosimilars. For content from the Biosimilars Council, you can visit our resource page, but we encourage you to review some of AAM’s most relevant resources below – including data on how generic medicines can increase patient access to needed medicines, why generic medicines are essential to lowering health care costs, and how you can encourage your member of Congress to act in support on generic and biosimilar medicines.
For more information or to view AAM’s full resource library, we encourage you to take a visit the AAM website.

Access and Savings Report
Anybody who cares about the U.S. health care system, and all the patients who depend on it, should recognize the role that generic and biosimilar medicines play in keeping people healthy and productive. AAM believes that this report will inform the conversation, in Washington D.C. and in state capitals, about how to rein in prescription drug prices. To accomplish our shared national goal we need to keep medicines accessible. Preserving and, indeed, expanding generic savings must remain a priority.
AAM White Paper: Ensuring the Future of Accessible Medicines in the U.S.
Patient health and well-being depends on the uninterrupted availability of lower-cost generic and biosimilar medicines. Moreover, as patients live longer the importance of a robust and sustainable generic and biosimilar medicines industry becomes only that much more important. Policymakers must act quickly to ensure continued saving and market-based competition, as well as prevent shortages, for future availability of affordable medicines. This requires:
- Enactment of the CREATES Act to prevent regulatory shenanigans;
- Scrutiny of patent gamesmanship to ensure that generics and biosimilars are able to launch at the earliest possible date;
- Continued regulatory attention to the review and approval of complex generics;
- Placement of biosimilar medicines on a level competitive playing field;
- Repeal or modification of the Medicaid Generics Penalty to prevent unintended harms;
- Focusing state drug pricing efforts on high-priced brand drugs that drive costs; and
- Increasing use of cost-saving generics for low-income patients in Medicare.
Altogether, these will ensure that generic and biosimilar medicines can enter new markets and that such markets are sustainable for the long-term.
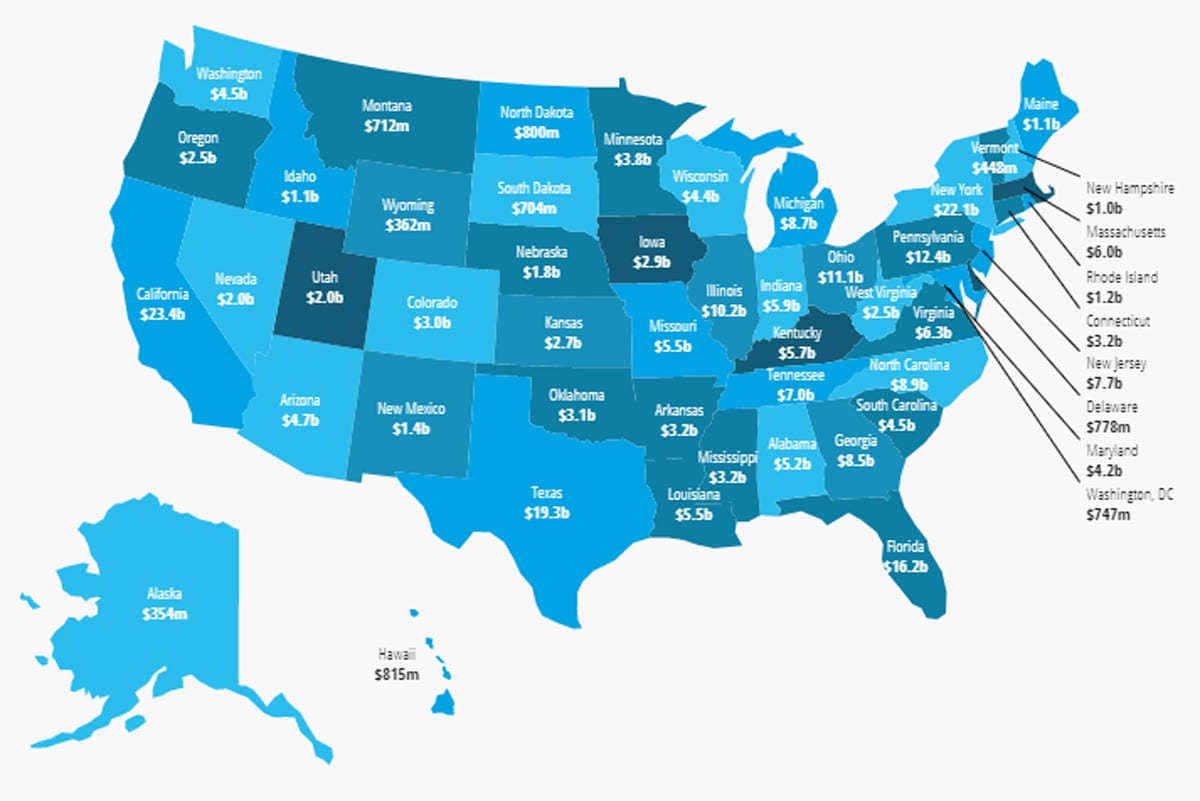
2018 Access and Savings Report – Interactive Savings Map
In 2017, the per-state average savings realized by using generic prescription drugs was $5.2 billion. The statewide annual amount saved ranged from $354 million in Alaska to a high of $23.4 billion in California. The Association for Accessible Medicines has brought together state level savings information into an easy to use interactive map, where you can view the breakdown of savings from generic medicines by state, savings type, and more.