This annual report is produced by the IQVIA, the standard-bearer for measuring data, pharmaceutical use and spending in the United States.
2017 Generic Drug Access and Savings in the U.S. Report
Healthcare costs continues to dominate national headlines and to feature prominently in the current political debate. That’s why the release of the Association for Accessible Medicines (AAM) Generic Drug Access and Savings in the U.S. Report is more timely than ever.
Compiled by the QuintilesIMS Institute, the ninth annual edition of the report details the savings generics provide across multiple segments, including by therapy area, payer type, patient demographic, and state.
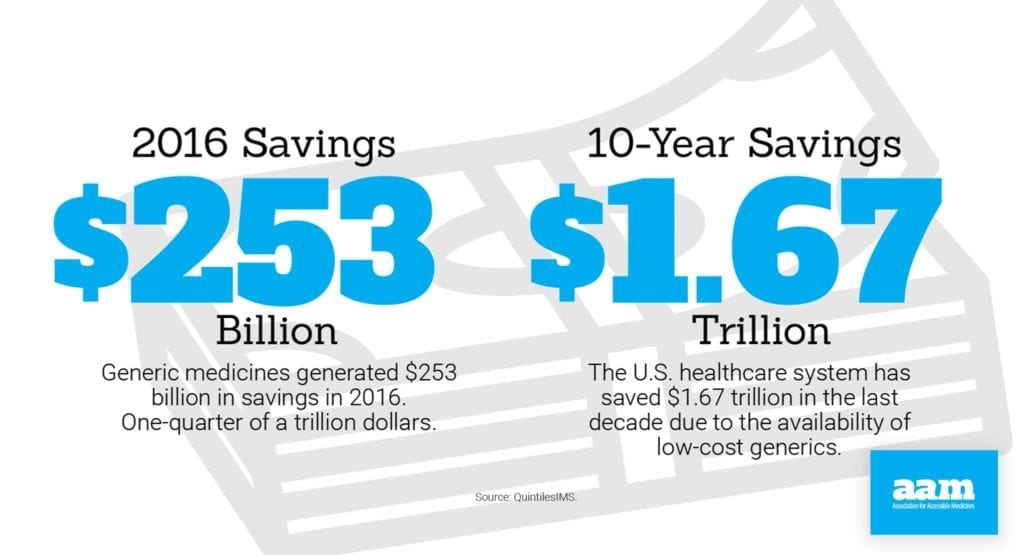
In today’s fluid healthcare economy, it is important to distinguish between the factors contributing to cost increases and those that drive savings. Take, for example, specialty drugs, a class of prescription drugs generally used to treat complex diseases. Today, branded specialty drugs comprise 1% of prescription drugs prescribed but remain responsible for more than 32% of total drug spending, according to separate QuintilesIMS data.
Biologics, which are drugs derived from living cells, are often classified as specialty drugs. These are medications you may see advertised on television in commercials many times a day. They are often infused or injected and are typically very expensive. Biosimilars are safe, effective and less costly alternatives to brand biologic medicines used in the treatment of cancer, rheumatoid arthritis and other conditions.
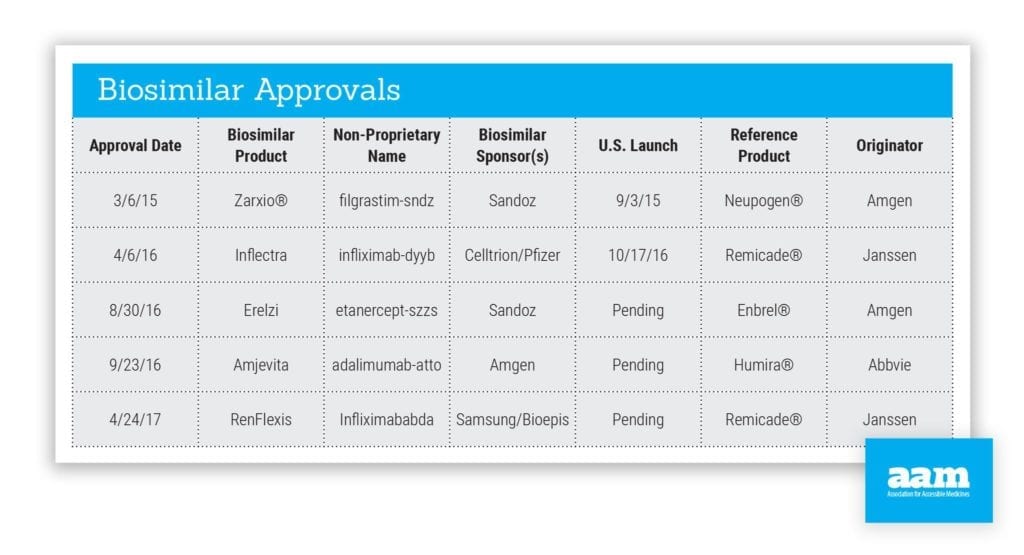
Biosimilars provide more competition in the market, enabling patients, payers, physicians and others to benefit from greater choice at lower costs when it comes to treatment options. Current estimates suggest that biosimilars could save consumers as much as $250 billion in the next 10 years.
The new report breaks down how generics and biosimilars fit in to the U.S. pharmaceutical ecosystem. The data demonstrates how important generic and biosimilar medicines are now and will continue to be in containing our nation’s increasing prescription drug costs.
“The results of the report speak for themselves: generics and biosimilars don’t just put your medicines within reach, they put the rest of your life within reach as well.” – Christine Simmon, Executive Director
Patients featured throughout the report rely on generics to maintain their families’ health and use savings from generics to afford other everyday needs like paying for rent and groceries. Without generics and biosimilars, many Americans — our family, friends and neighbors — would face incredibly difficult choices between their health and life’s other essentials. That’s why we believe that manufacturers, government and regulatory groups must work together to create policies that create competition and prioritize patient access.
If you’re interested in learning more about biosimilars, join us at our annual conference, Leading on Biosimilars. Reserve your spot here: http://biosimilarscouncil.org/leading-on-biosimilars
The Biosimilars Council is now on Twitter, Facebook, and LinkedIn, follow us for the latest updates on biosimilars.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 23 percent of total drug spending. Additional information is available at www.accesiblemeds.org.
