Although biosimilars have made great strides in the last year, there remains much work to be done. This point is made clear in the new U.S. Generic and Biosimilar Savings Report, issued by the Association for Accessible Medicines and its Biosimilars Council. Biosimilars made important progress in 2021 – increasing savings for patients, lowering overall prices, and expanding patient access to therapy. But despite this, barriers continue to prevent patients and taxpayers from reaping the full rewards of lower-cost biosimilar medicines.
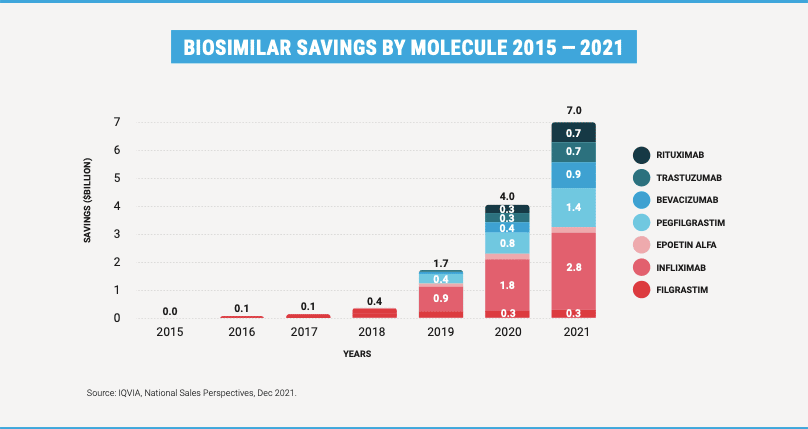
In 2021, the use of biosimilars generated more than $7 billion in savings, more than 50 percent greater in just one year. These savings were driven by increased adoption, as well as lower prices. Today, the average biosimilar is priced 50 percent lower than the brand was priced when the biosimilar came to market. And brands are also reducing their prices in response to biosimilar competition. This means that all patients benefit from biosimilar competition, even those not using a biosimilar.
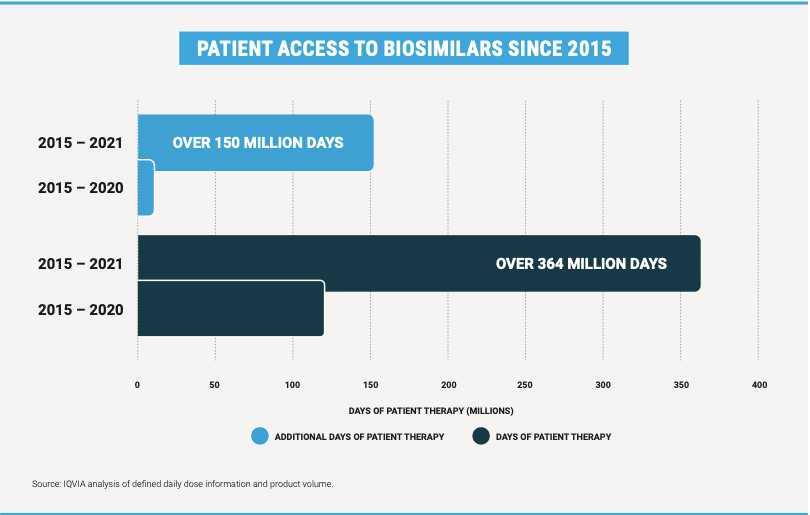
More importantly, biosimilars continue to provide safe and effective treatment options for patients. To date, biosimilars have been used in more than 364 million days of patient therapy. And independent consulting firm IQVIA, acting on behalf of the Biosimilars Council, determined that overall patient therapy has increased by more than 150 million days of therapy. This means that patients are receiving therapy they otherwise would not have received without biosimilar competition.
But biosimilar adoption continues to face challenges. Misinformation about the safety and efficacy of biosimilars continues to be an obstacle to use. Patent thickets have delayed biosimilar launches. And brand drug rebates and warped reimbursement incentives can favor use of higher cost brands and limit patient access to lower cost biosimilars.
The Biosimilars Council continues its work to help biosimilars overcome these obstacles. The Biosimilars Patient Resource Center and collaboration with other professional associations on educational materials, are key tools to fight misinformation. And the Council continues work to ensure that patients have access to a robust biosimilars marketplace.
Looking forward, next year will bring heightened attention to biosimilars as new biosimilars for the treatment of arthritis, Crohn’s disease, and other autoimmune conditions enter the market. It is critical that policymakers work with health plans, PBMs, providers and patients to encourage access to and use of lower-cost biosimilars. Only then will patients and taxpayers experience the full value and savings from biosimilar competition.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.