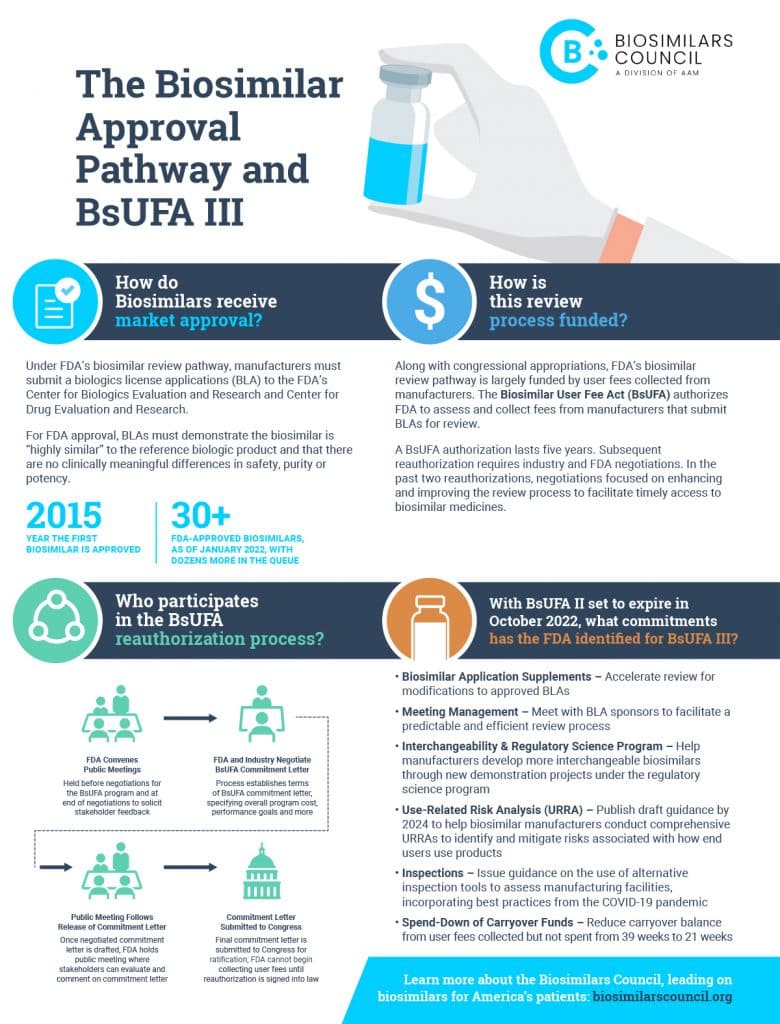
This year, Congress will consider legislation that codifies performance goals for the review of drug applications by the U.S. Food and Drug Administration (FDA). In exchange for agreeing to enhancing its review of drug marketing applications, the FDA is able to collect user fees to fund many of its review activities, which benefits patient access to medicines. This legislation must be reauthorized every five years, and the process is known as the reauthorization of “user fee acts.” The Biosimilar User Fee Act, or BsUFA, will include a commitment by biosimilar manufacturers to pay fees that support the FDA’s review of biosimilars, as well as other regulatory activities. For example, BsUFA fees accounted for 60% of total program costs in 2020, and it is critical that a reauthorization is signed into law before the end of the year, when the current funding agreement expires.
BsUFA Reauthorization and Modernization Is the Key to a Healthy Biosimilar Market
In anticipation of this reauthorization, FDA has held numerous public meetings with stakeholders to solicit feedback about the terms between biosimilar manufacturers and the FDA, which culminates in what is known as the FDA’s performance goals agreement. The Biosimilars Council was one of the principal negotiators throughout this process and advocated for enhancements to the biosimilars review pathway.
FDA-approved biosimilars are highly similar to and have no clinically meaningful difference from the brand products they reference. Like generics, biosimilars compete with brand products, increasing patients options for care and lowering their costs. BsUFA will modernize and streamline the biosimilar approval process to help ensure patients have access to lower-cost options for their care, including:
- Making meetings between FDA and biosimilar manufacturers more efficient, targeted, informative and clear;
- Creating new demonstration projects for interchangeable biosimilar manufacturing and informing a comprehensive strategy for advancing interchangeability and future guidance development;
- Issuing guidance to help clarify existing processes and gather new data on how end users (patients or doctors) use biosimilars;
- Publishing guidance on the continued use of remote inspection tools for manufacturing facilities used during the COVID-19 pandemic; and
- Strengthening the Agency’s information technology capabilities to better support biosimilar reviews.
In Congress, BsUFA and the other user fee reauthorizations have typically enjoyed strong bipartisan support due to the rigorous process for FDA and many stakeholders to provide input into improving the FDA’s review processes. Once signed into law, this will be the third biosimilars user fee agreement. It is imperative that Congress move swiftly to reauthorize this bill to ensure the sustained growth of biosimilars and the savings they provide for patients.
For more information about BsUFA, click here.
To learn more about other advocacy involving biosimilars development, view our advocacy page.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.