We’ve just completed this year’s GRx+Biosims 2020, the premier scientific and regulatory event for the U.S. generics and biosimilars industries, and are excited to recap this successful first-ever virtual event. This year, in line with 2020, the program featured important sessions on the response to the COVID-19 pandemic and industry growth, as well as critical discussions about the future of the biosimilars market in the U.S. and abroad. Top officials and subject-matter experts spoke on the role of generic and biosimilars on increasing patient access to medicines during a global pandemic; generic and biosimilars development; the regulatory process; and the evolving policy landscape.
GRx+Biosims 2020 Conference Wrap-Up Blog
Missed the conference?
You can still watch sessions from industry experts and key influencers on-demand.
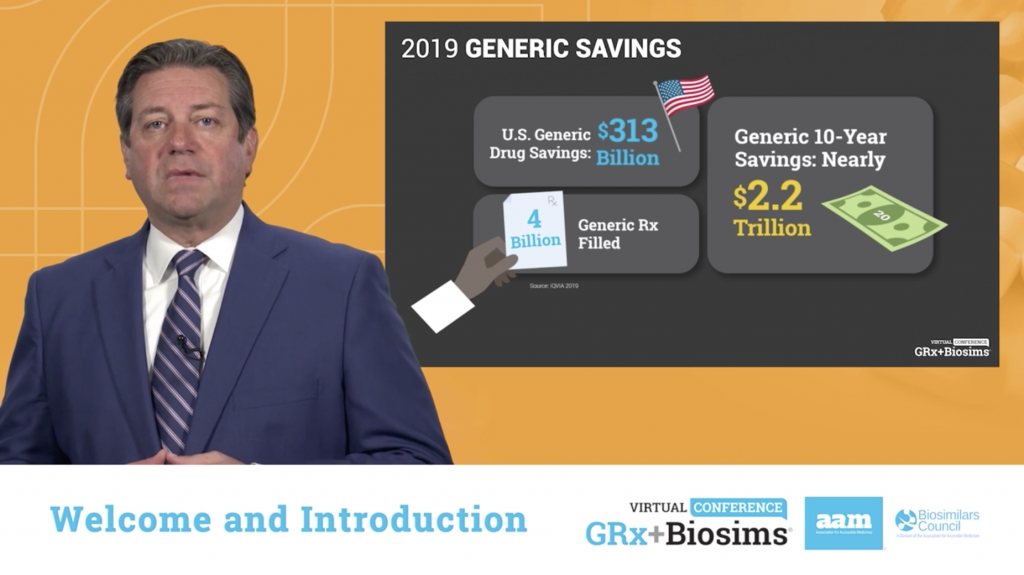
The virtual conference opened with a welcome from AAM President and CEO Dan Leonard, who expressed optimism for the future of America’s generics and biosimilars industry.
“I think we are in an enviable position,” Leonard said, addressing more than 400 conference attendees representing the many facets of America’s health care system. “There are plenty of challenges and obstacles – and they are significant – but without changing who we are or what we do, we are the answer to the questions so many policymakers in Washington and in state capitols are facing.”
Conference sessions continued, featuring a robust line-up of prominent decision-makers and stakeholders. U.S. Food & Drug Administration (FDA) Commissioner Stephen Hahn, M.D. delivered the first keynote address. He spoke of the contributions the generics and biosimilars industry has made, saying:
“We need to continue our collaborative efforts to contain the spread and to find new treatments and vaccines. I want to thank the AAM for your response to this public health emergency. I know that many companies in the U.S. generics and biosimilars industry have played an important role as partners in these efforts. We appreciate your blueprint outlining incentives for ways to diversify the supply chain and enhance manufacturing of pharmaceuticals in the U.S. These are essential aspects of the collaboration necessary to defeat this virus.”
Speaking about the supply chain, drug developments and application processes amid COVID-19 disruption, Dr. Hahn assured attendees that FDA is “adapting to the current challenges and applying what we have learned to carry out our responsibilities.” Specifically, FDA “has issued more than 60 related guidance documents and has revised COVID-19-related guidance documents to provide updated policies, transparency and regulatory flexibility to address the vital medical products and public health issues facing the American public during this pandemic.”

Dr. Hahn was followed by other notable keynote addresses from the Center for Drug Evaluation and Research (CDER) and the Office of Regulatory Affairs. CDER’s Patrizia Cavazzoni, M.D., and ORA’s Elizabeth Miller, PharmD, touched on their department’s action during the pandemic, the challenges and solutions in upholding quality standards throughout the global supply chain and the growth of the generics and biosimilars markets. They stressed the importance of global collaboration in ensuring that patients everywhere are able to access the medicines they need.
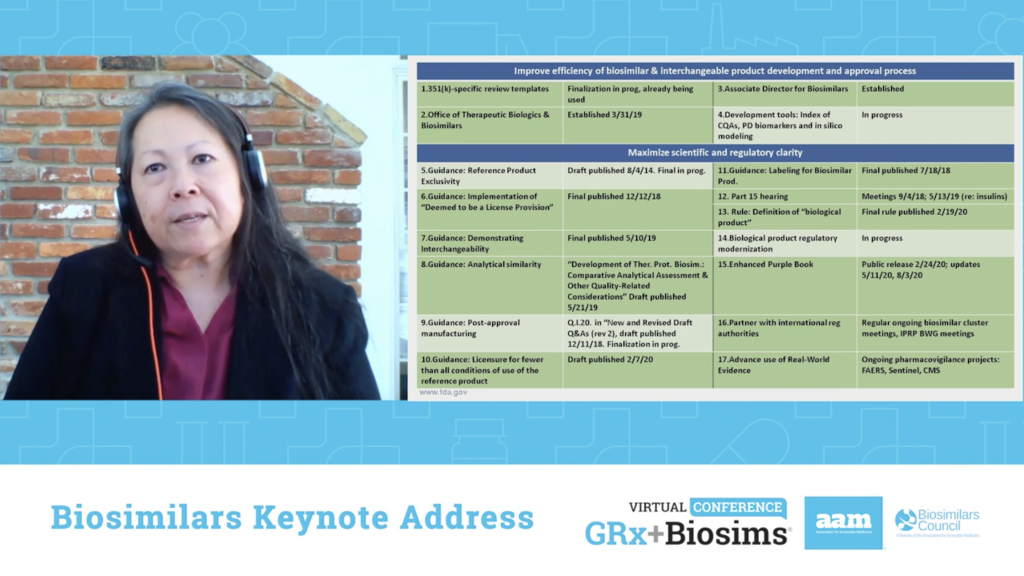
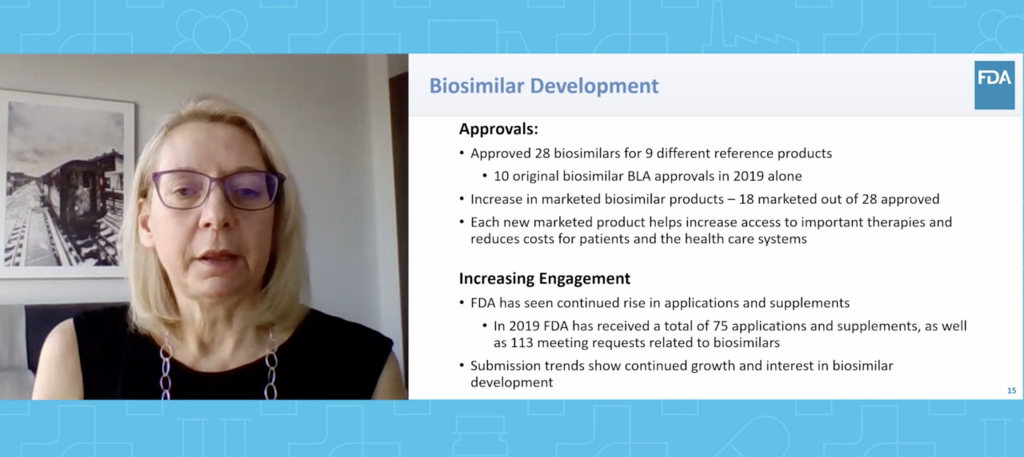
“Most health-related organizations have an aligned mission on wanting to do the best for patients while minimizing their risk and maximizing their benefit. This is nothing new,” said FDA Director of the Office of Therapeutics Biologics and Biosimilars Sarah Yim, M.D. in her keynote address about the state of biosimilars. “However, it’s surprisingly easy to lose sight of the mission and make decisions that aren’t consistent with it in the clamor of the day to day…Our words, actions and decisions with respect to biosimilars should be clear on how they fit with the mission and be consistent with the overall objective of biosimilars improving patients’ health and health care.” Dr. Yim then outlined FDA’s progress on the Biosimilars Action Plan, the goals accomplished and the work that still needs to be done.

***
Related Resources
FDA Biosimilar Approvals and Launches
Jeni Got Her Life Back, Thanks to a Biosimilar
***
With a nod to this work, many focused on the critical steps that must be made to ensure biosimilars market adoption for the benefit of patients. Specifically, multiple panels with Biosimilars Council members covered the political landscape in the U.S. and its potential effects on the biosimilars market in America. One panel led by AAM Senior Vice President for Policy and Executive Director of the Biosimilars Council Christine Simmon examined the decision regarding the Affordable Care Act (ACA) and how different scenarios may affect the pathway for biosimilars through the Biologics Price Competition and Innovation Act (BPCIA). Featuring panelists including Lauren Aronson, Partner at Mehlman, Castangnetti, Rosen & Thomas and Chad Landmon, J.D., Partner at Axinn, Veltrop & Harkrider LLP, the discussion surrounding the many ramifications of the Supreme Court’s forthcoming decision on the future of the ACA and the related biosimilars provision.
This year’s virtual conference saw more than 400 participants from stakeholder groups including government, patient groups, and generic and biosimilar companies come together to discuss important issues facing the industry. Ultimately, while this year has brought new challenges, GRx+Biosims affirmed we should remain optimistic about the future of biosimilars and generics in the U.S.
Thank you again to everyone who spoke and attended. If you couldn’t make it to the GRx+Biosims virtual conference, you missed out on some timely, insightful and incredibly beneficial content for everyone in the generics and biosimilars industry. The good news is that you can still access the conference platform and purchase key sessions to watch on-demand.
To stay up to date on the latest biosimilars news and resources, follow Biosimilars Council on social media and subscribe to our newsletter.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.
