Biosimilars provide more options for care at a lower cost for patients, but due to Medicare’s perverse incentives, many PBMs and health plans have been slow to prioritize biosimilars, leavings savings on the table. Last week, the HHS Inspector General (OIG) released an important analysis that drives home just how poorly Medicare plans are doing when it comes to making FDA-approved biosimilars available to America’s seniors.
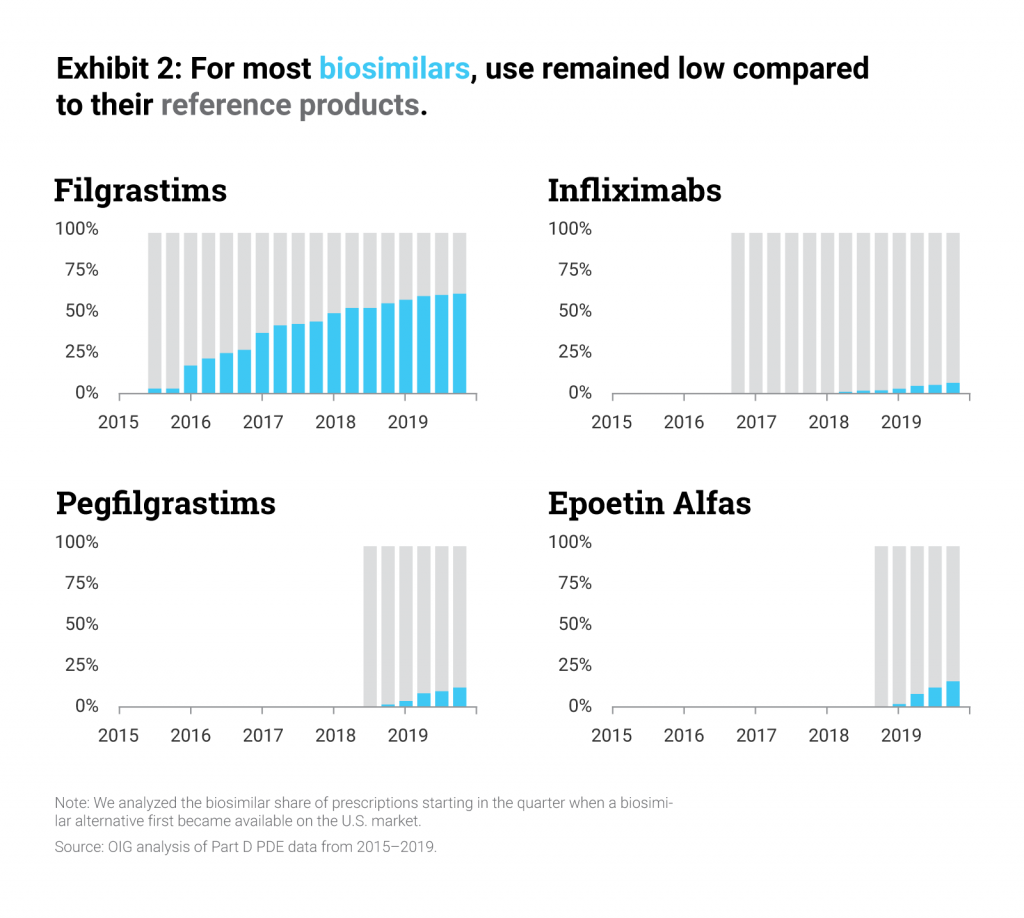
Specifically, the report found that biosimilars were used much less in the Part D setting than their reference products, accounting for fewer than 1 out of every 5 prescriptions for biosimilars and their reference products in 2019. A major factor in this shortfall was the fact that many plans did not cover biosimilars at all, and those plans that did cover biosimilars rarely designed their formularies in such a way as to encourage use of lower-cost biosimilars over the reference biologic.
The OIG calculates that the result is millions of dollars in lost savings for patients and taxpayers. The report found that, in 2019 alone, the Medicare program could have saved between $84 million and $143 million, or 18% to 31% of the program’s gross spending on biosimilars and branded products. And OIG determined that seniors in Medicare missed between $2-$3 million in savings, which is 12% to 22% of seniors’ spending on these products.
The OIG recommended that the Centers for Medicare & Medicaid Services (CMS) encourage Part D plans to increase access to biosimilars and that it monitor plans’ coverage of biosimilars, recommendations that the Biosimilars Council has long supported. CMS took an important step in this direction in 2020, when it allowed plans to use a second, “preferred,” specialty tier with lower cost-sharing for seniors. Unfortunately, in practice, few plans have used this flexibility to reward patients for using lower cost biosimilars.
There are a number of options for CMS and Congress to encourage greater biosimilar adoption in Medicare Part D.
- Congress can update the design of the program to shift greater liability to health plans and encourage them to prioritize use of lower cost biosimilars and generics. Increasing plans’ liability for higher cost brands compared to lower cost generics or biosimilars in the catastrophic phase would muffle the impact of rebating, which exposes seniors to higher costs at the pharmacy counter.
- Congress can pass the bipartisan Ensuring Access to Lower-Cost Medicines for Seniors Act, H.R. 2846, to reduce the out-of-pocket costs for seniors by requiring coverage of newly approved biosimilars and creating a dedicated specialty tier.
- CMS can clarify that all biosimilars are eligible for non-maintenance mid-year formulary changes, which would give plans additional flexibility to make biosimilars available to patients more quickly.
The work to encourage biosimilar adoption in Part D is just beginning and will grow in importance as more biosimilars enter the Part D market. In particular, the entry of as many as seven biosimilar versions of Humira (adalimumab) next year represents an important test for the program. It is critical that the Administration and Congress take advantage of these bipartisan approaches to reduce drug spending by increasing the use of biosimilars and ensure that their savings potential is fully realized for patients.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.