As policymakers and patients wrestle to contain drug spending, a recent report from the IQVIA Institute for Human Data Science shows how biosimilars are fulfilling the hope of lower prices, with potential five year savings exceeding $180 billion. But the same report highlights threats to making this hope a reality.
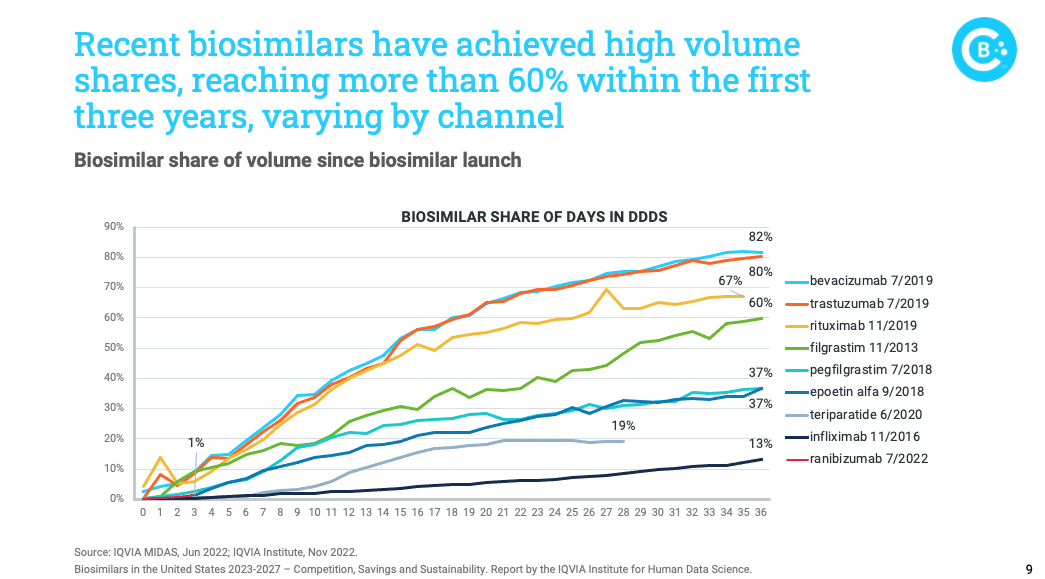
Adoption of biosimilar medicines has continued to rise, with some recently launched biosimilars showing impressive growth. However, not all biosimilars have achieved this same level of market share, illustrating adoption challenges that demand attention.
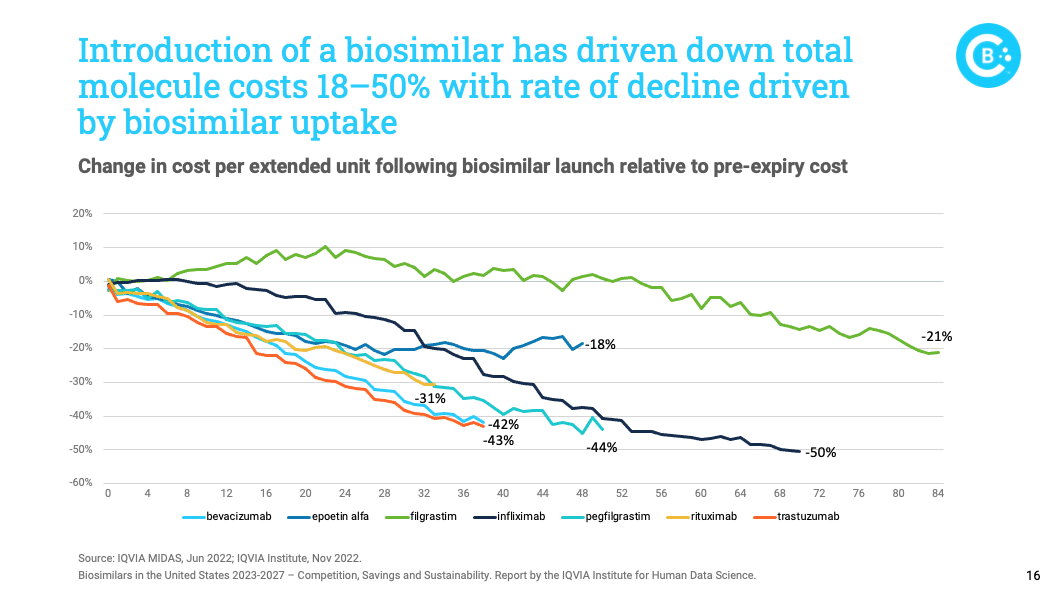
The report finds that biosimilars are not only bringing lower prices, but they are driving down the prices of brand biologics. For instance, the average price of infliximab biologics and biosimilars has declined by a staggering 50 percent.
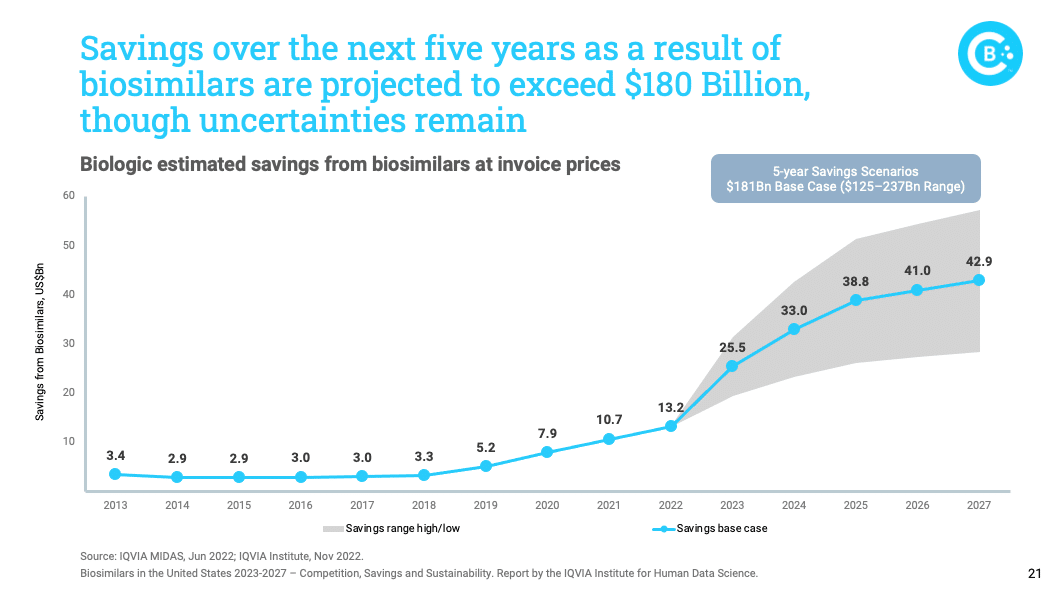
In fact, overall savings generated by biosimilars could exceed $180 billion by 2027. With nearly half of US drug spending coming from expensive biologics, these savings are critical to the overall sustainability of our healthcare system.
Unfortunately, many patients are denied access to these savings. Policy and reimbursement incentives can encourage providers and pharmacy benefit managers (PBMs) to prefer high-cost brand biologics. And even when drug formularies cover biosimilars, the savings are often retained by PBMs and not shared with patients. These threaten the sustainability of long-term biosimilar competition.
Even when biosimilar versions of insulin or adalimumab are priced at large discounts, many PBM formularies continue to prefer higher-priced brand biologics with high rebates. These practices delay patient access to lower costs and primarily benefit pharmacy benefit managers and health plans, not the patients they serve.
We are at a critical juncture in the development of the biosimilar market. We’ve seen significant progress in market growth and associated savings, but future progress depends on the policy decisions we make today. It is critical that policymakers take steps to encourage rapid adoption of lower-priced biosimilars and ensure that every patient has access to the treatments they need.
Join the Council’s monthly bulletin so you don’t miss future installments and to stay up to date on the latest developments in the biosimilars industry.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 20 percent of total drug spending.