Friends,
Right now, the biosimilars market is at a major inflection point, with momentum building in Congress for new drug pricing legislation while new biosimilars of America’s bestselling biologic medicine have come to market. While the summer heats up and folks head out on their vacations, the Biosimilars Council remains hard at work advocating for increased patient access to biosimilars and the savings they provide.
Here are some important updates for you.
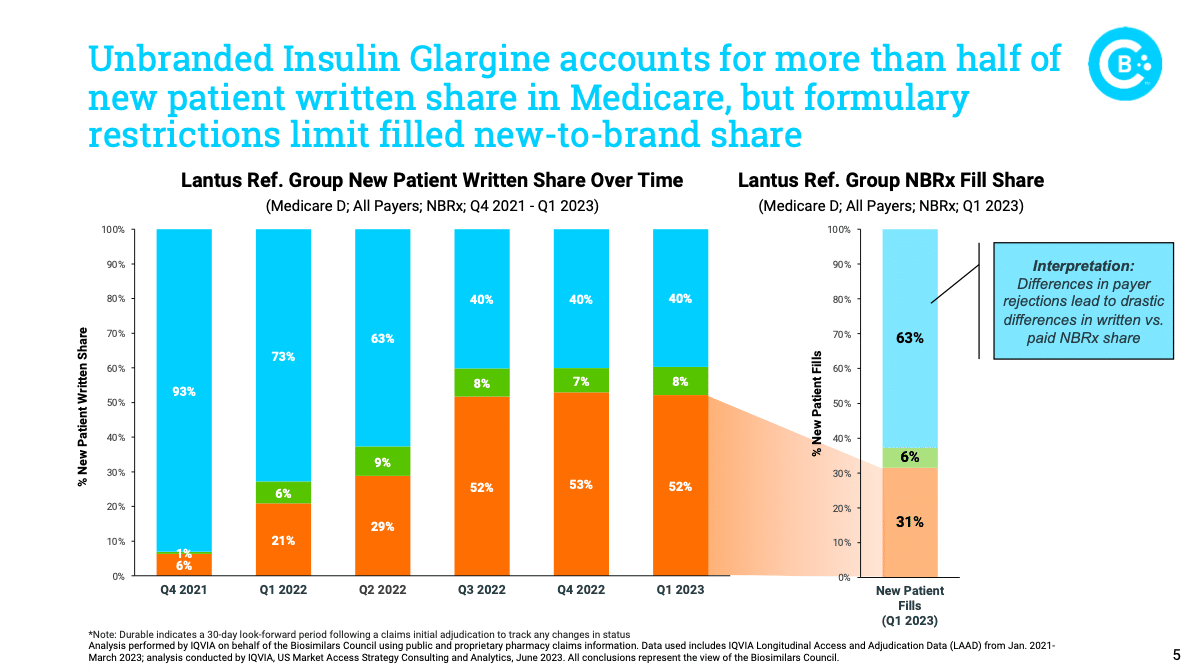
Yesterday, the Biosimilars Council released a new report tracking the uptake of biosimilar insulin products. Based on data from IQVIA, the report found that while demand for unbranded biosimilar insulin products has risen dramatically, patient access has been slowed by payer controls and formulary structure – particularly within Medicare. This report demonstrates the to reduce barriers to biosimilars, so that patients benefit from lower-cost treatments.
Read our press release and blog on the new insulin glargine report.
Coming out of the July 4th congressional recess, Senate Majority Leader Chuck Schumer (D-NY) reiterated that drug pricing legislation is a top priority for the chamber, with hearings and markups on legislation coming soon. David Gaugh, Interim President & CEO of the Association for Accessible Medicines (AAM), penned an op-ed in RealClearPolicy last month warning that Congress may be ignoring the price-reducing effect that biosimilar competition has on the market. His piece urges lawmakers to eliminate the perverse incentive structure that keeps prices high as they develop new drug pricing legislation this session.
For more details about how biosimilars promote savings, and the challenges currently inhibiting greater biosimilar uptake, check out our recent blog.
Additionally, July saw the introduction of eight new biosimilar versions of Humira (adalimumab). While these biosimilars will eventually deliver significant savings for patients, it will take some time for the market to mature enough for savings to materialize. Further, perverse incentive structures will stifle more rapid uptake unless Congress passes legislation to increase biosimilar access.
Despite the challenges we face, the biosimilars market has never been more vibrant, and delivered more savings, than it is right now. Later this year, AAM and the Biosimilars Council will release our annual savings report to illustrate the rapid growth of savings over the past year – stay tuned for more updates.
This summer is set to be a major milestone in biosimilar development, and we will continue working to ensure that biosimilar access continues to expand and deliver on its promise of providing significant savings for patients. For up-to-date information, resources, blogs, and research, be sure to check out our website.
-Craig

Encourage the adoption of biosimilars in the U.S. by forwarding this newsletter to a colleague or friend. They can subscribe here.
Want to hear directly from Executive Director Craig Burton?
Follow him on LinkedIn
Interested in joining the Biosimilars Council?
Contact Jewel Smith (jewel.smith@accessiblemeds.org) for more information