Biosimilar medicines are increasingly becoming an essential fixture in health care, reducing prices and increasing patient access. This is especially true for oncology treatment, where biosimilar medicines are helping expand access to care for cancer patients across the country.
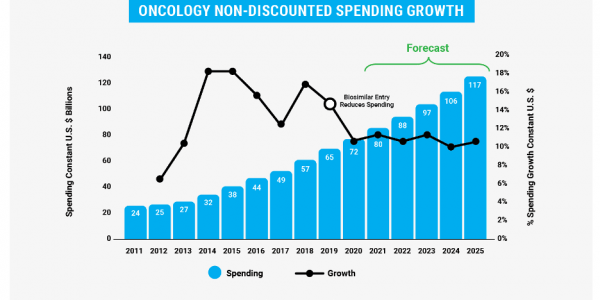
The 2021 Generic and Biosimilar Medicines Savings Report illustrates the growth in utilization of oncology biosimilars and resulting savings for patients. In 2020, increased adoption of biosimilars bent the curve in oncology spending, cutting the growth rate roughly in half since 2019. Together, biosimilar and generic medicines generated $16 billion in oncology savings in 2020, up from about $13.5 billion in 2019.
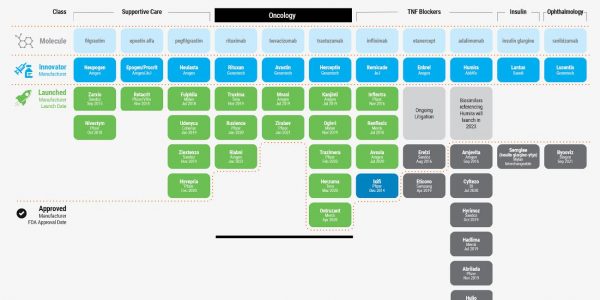
Biosimilars are used to treat brain, breast, cervical, colorectal, kidney, lung, and stomach cancers, as well as non-Hodgkin lymphoma. Biosimilars are also used in the supportive care that oncology patients receive so that they can maintain their white blood cell count and fight off infections.
The effect can be lifesaving for patients and their families. With greater access to biosimilar oncology medicines, patients can afford the treatments they need.
Helen, for example, went through years of trial-and-error with several different treatment options before turning to biosimilars. The change made all the difference for Helen, who said that “after just two treatments of the biosimilar I started to feel like me again. I was able to go back to work and my most important roles as a mother and wife.” (Follow the link to learn her full story)
The Biosimilars Council has been steadfast in our commitment to helping cancer patients like Helen through the promotion of biosimilars. We are proud to have worked with organizations like the American Cancer Society Cancer Action Network (here and here) to educate cancer patients about biosimilars and make their treatment options more accessible and affordable, as well as to advocate for policies that encourage biosimilars utilization.
The BIOSIM Act, for example, would help increase physician reimbursement for biosimilar medicine. This bill would increase reimbursement for biosimilars by 2% to ASP+8% and would apply only when the biosimilar’s ASP is lower than the brand-name biologic’s ASP. This incentivizes the use of biosimilars, which have an average 30% lower ASP compared to the respective reference biologic.
Similarly, the Increasing Access to Biosimilars Act would direct the Centers for Medicare & Medicaid Services to establish a voluntary, national demonstration project under Medicare Part B to determine the benefits of a shared savings payment for biosimilars. Participating providers would receive a portion of the savings for prescribing a biosimilar with a lower ASP than the reference biologic. This program is designed so that savings are guaranteed for Medicare and taxpayers.
As we come together to mark Breast Cancer Awareness month, we urge policymakers to support these bills. With their help, we can ensure that cancer patients can afford the treatments they need.