test page
Biosimilars Timeline
-
2010 Passage of BPCIA

The Biologics Price Competition and Innovation Act (BPCIA), enacted in 2010, provided a framework for biosimilars approval, adoption and access in the U.S. How did we get there?
- 1980s: The first biological medicinal products produced by DNA recombinant techniques are approved
- 1986: First monoclonal antibody receives FDA approval
- 1998: First biologic for rheumatoid arthritis is introduced: Omnitrope
-
2014 First FDA Guidance Issued

Guidance documents represent FDA’s current thinking on a topic. The FDA’s first guidance on biosimilars was issued in 2014 on the Development and Approval of Biosimilar Products in the U.S., creating clear regulatory expectations for biosimilar products. This guidance provides information on developing biosimilarity, safety and efficacy.
-
2015 First U.S. biosimilar approved: Zarxio
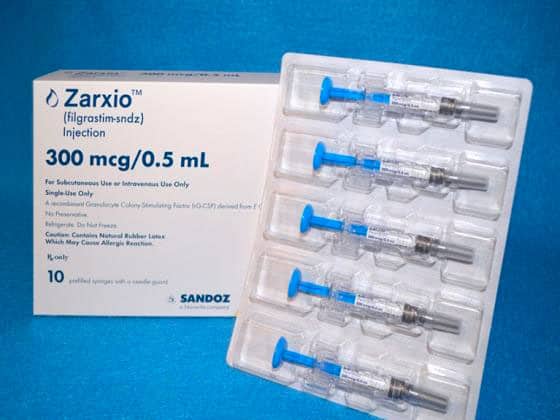
In March 2015, the FDA approved its first biosimilar, Zarxio (filgrastim-sndz) for patients with cancer receiving chemotherapy and radiation to support white blood cell creation. Sandoz was the first company to receive approval of a biosimilar in the U.S. through the new FDA biosimilars pathway established under the Biologics Price Competition and Innovation Act.
-
2015-2020 Increasing Access through Biosimilar Policy

Congress and multiple government agencies have played a large part in the growth of biosimilars. Through continuing to issue FDA guidance for industry, providing clearer guidelines on biosimilar approval and creating new pathways for biosimilar access and reimbursement, government agencies have helped paved the way for biosimilar success.
- 2015: FDA Final Guidance issued on demonstrating biosimilarity with a reference product
- 2016: FDA Final Guidance issued on naming and labeling for biosimilar products
- 2017: Centers for Medicare & Medicaid Services issues policy on biosimilars reimbursement, giving each biosimilar its own average sales price.
- 2019: Bipartisan members of Congress introduced multiple pieces of legislation to incentivize biosimilar use and adoption
-
2018 RAND Report Projects Billions in Savings

U.S. research institution RAND report finds that biosimilars could save the U.S. health care system more than $54 billion in savings over the next 10 years and offer access for needed treatments to over 1.2 million patients. -
2018 A Push for Patient Access

Momentum builds for more biosimilar approvals and greater access and to fight anticompetitive tactics from brand biologic companies. With the creation of the Biosimilars Action Plan in June 2018, FDA pledges to propel biosimilars forward for the sake of America’s health care system and patients. -
2019 A Watershed Year for Biosimilars
 A record 10 FDA biosimilar approvals and increased uptake and utilization rates by payers characterize the most concrete progress yet.
A record 10 FDA biosimilar approvals and increased uptake and utilization rates by payers characterize the most concrete progress yet.-
- Record number of FDA approvals: 10
- Record number of products launched
- May 2019: FDA Final Guidance issued on interchangeable biosimilars
- November 2019: FDA issues draft guidance on insulin biosimilars
- October 2019: World Health Organization (WHO) and International Generic Biosimilar Medicines Association (IGBA) sign memorandum of understanding to encourage and promote biosimilars access worldwide
-
-
The Next Ten Years A Bright Future for Biosimilars
 Biosimilar adoption in the U.S. health care system is within reach. To ensure continued success of biosimilars for the next 10 years and beyond is dependent on the decisions made today. While approvals have increased access and use have not.
Biosimilar adoption in the U.S. health care system is within reach. To ensure continued success of biosimilars for the next 10 years and beyond is dependent on the decisions made today. While approvals have increased access and use have not.Increasing biosimilar availability could provide as many as 1.2 million U.S. patients access to lower cost treatment options by 2025. The events of 2020 have made it clear that health care solutions are needed that improve patient access to medicines and increase savings for the entire U.S. health care system. Biosimilars are an essential part of this reform. Fulfillment of the biosimilar promise can happen if we educate the medical profession as well as federal and state policymakers.