Each month, the Biosimilars Council sends a newsletter with the latest updates in the biosimilars industry including policy movement, educational resources and upcoming events. To stay current on biosimilars, sign-up to receive these monthly updates on the form to the right.
Biosimilars Bulletin | November 2020
News and Updates
Biosimilars Council Joins IGBA in Celebrating Global Biosimilars Week
Affordable Care Act and the Future of Biosimilars
B10similars: Reflecting on 10 Years of Biosimilars
Why Severability Should Be Every Biosimilar Makers’ Rallying Cry In 2021
OTBB Director Discusses FDA’s Roadmap for Biosimilars
3 Reasons U.S. Payers Will Make 2021 a Banner Year for Biosimilars
Regulatory Policy May Not Stop Comparative Efficacy Trials
Managing Biosimilar Product Selection: Practical Considerations for Implementation of Biosimilars in Oncology
Insulin Biosimilars Market
Featured Resources

Lessons for the United States on Europe’s Biosimilar Experience

Biosimilars Approvals and Launches
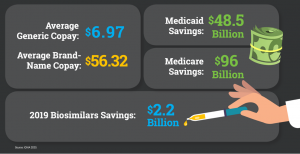
2020 Generic Drug & Biosimilars Access & Savings in the U.S. Report
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 23 percent of total drug spending. Additional information is available at www.accessiblemeds.org.
