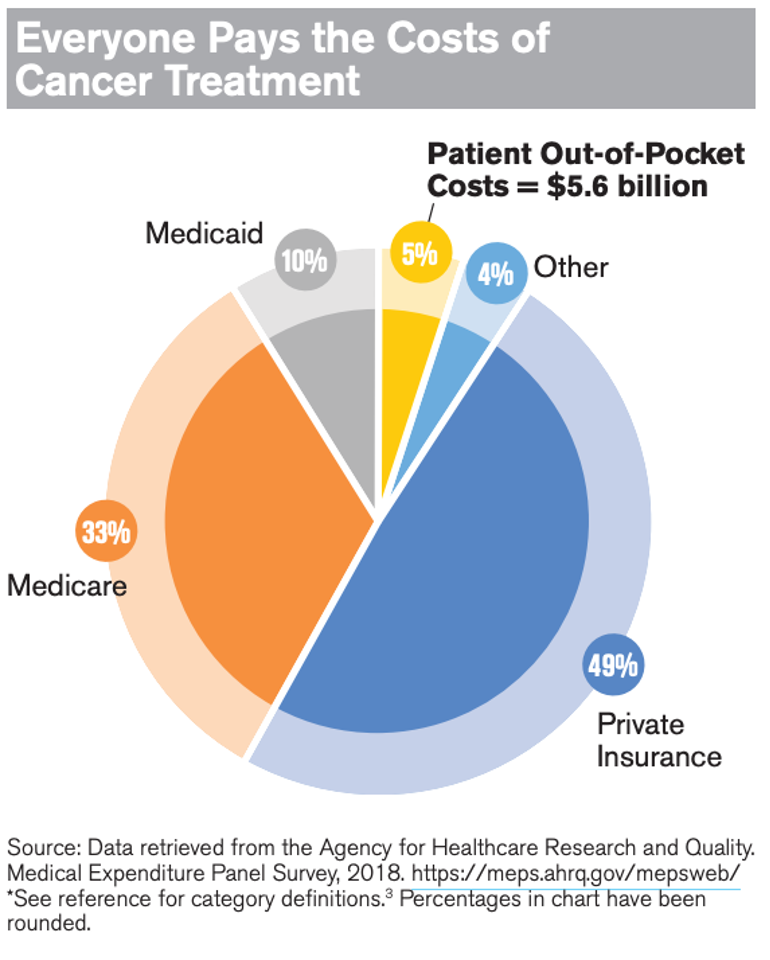
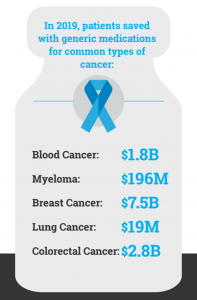
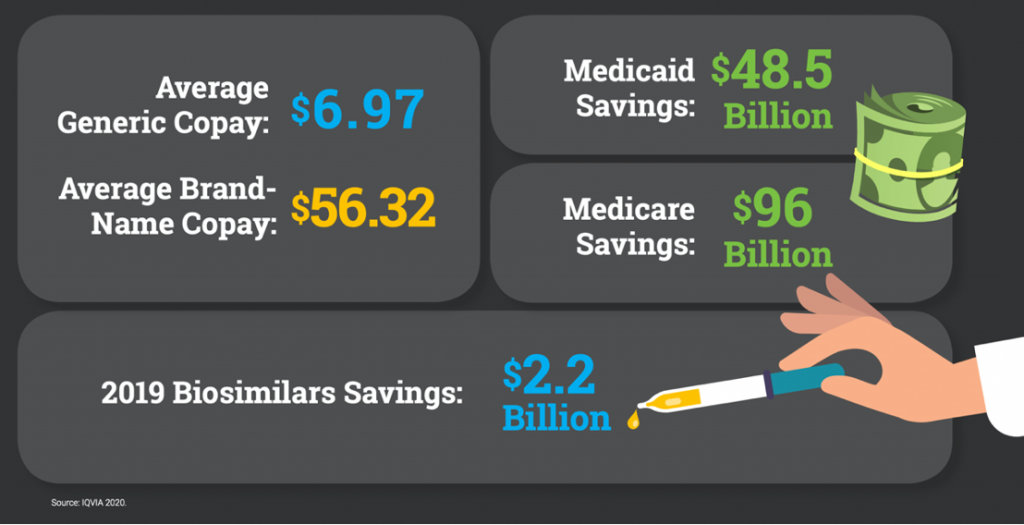
In 2020, cancer claimed the lives of 600,000 Americans. According to the American Cancer Society (ACS CAN), there are approximately 1.7 million new cases of cancer in the United States each year, and more than 16.9 million Americans have a cancer history.
Today, on World Cancer Day, AAM and the Biosimilars Council pause to recognize the toll cancer takes on our nation. Generic and biosimilar medications that treat cancer are improving the lives of patients and easing the financial burden on patients and their families. An ACS CAN report confirmed that patients and health care payers can save money when patients use a generic or biosimilars alternative to a branded drug. Non-Hodgkin lymphoma patient Helen discussed her experience with taking a biosimilar in this video, “Taking a biosimilar is easy. I just go to the doctor’s, get a shot and leave. After everything I have been through it’s wonderful.”