Each month, the Biosimilars Council sends a newsletter with the latest updates in the biosimilars industry including policy movement, educational resources and upcoming events. To stay current on biosimilars, sign-up to receive these monthly updates on the form to the right.
Biosimilars Bulletin | January 2021
News and Updates
Cancer Costs and Options for Care in the United States
AAM CEO Urges President-Elect Biden to Act on Access
Prescription Drug Policy: The Year in Review and the Year Ahead
Economists Probe Cost-Effectiveness of Biosimilars
Biosimilars in Cancer Care: Insights From 2020 and Expectations for 2021
Featured Resources
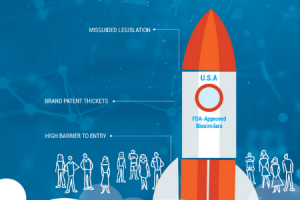
Patent Abuse Blocks Patient Access to Biosimilars

2020 Generic Drug & Biosimilars Access & Savings in the U.S. Report
Upcoming Events
GRx+Biosims 2021 | November 8-10, 2021
GRx+Biosims 2020 Virtual Conference
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available on our about page.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 23 percent of total drug spending. Additional information is available at www.accessiblemeds.org.
