Resources
Home / Resources
Providing the best advocacy resources for biosimilars.
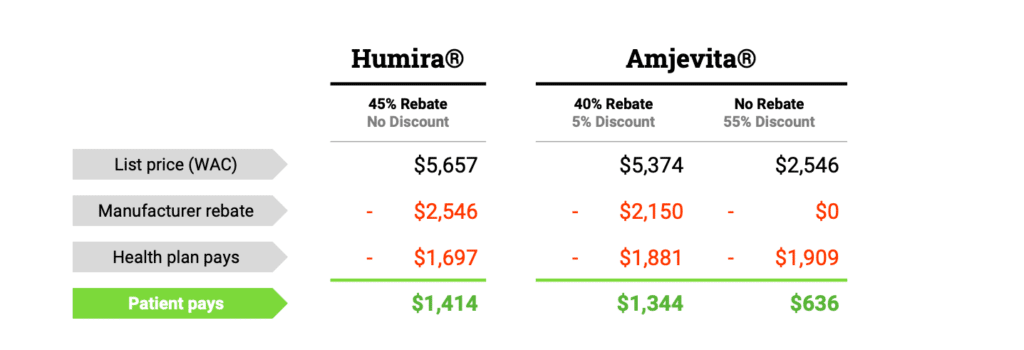
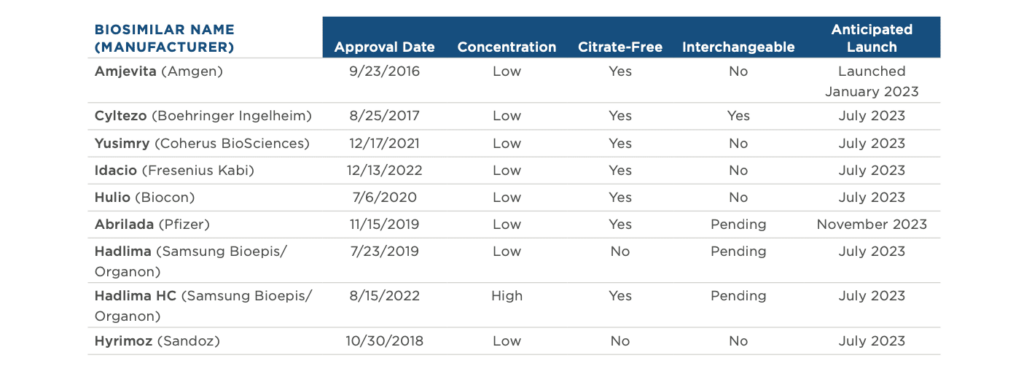
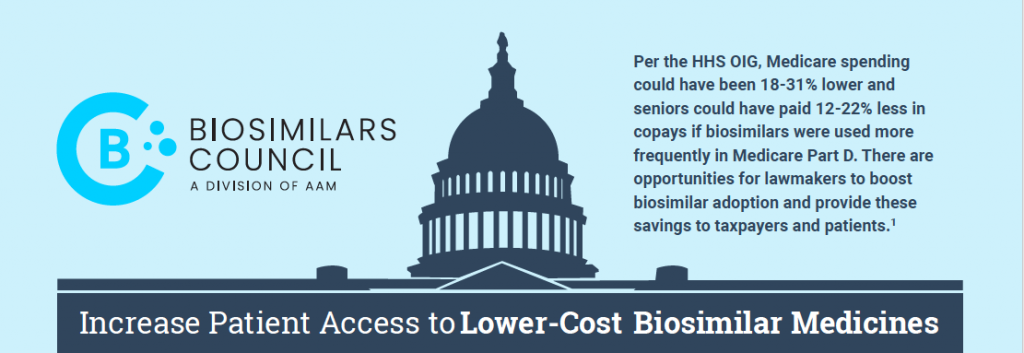
The resource center is an information hub—great for gathering scientific, historical and policy-focused facts on safe and effective biosimilar medicines that have saved America’s patients and the U.S. healthcare system $36 billion since 2015.
The Biosimilars
Patient Resource Center
The Biosimilars Patient Resource Center is a convenient site for answers to your pressing questions about biosimilar medicines.
Whether you are a current or potential biosimilar or biologics patient, a caregiver, a medical professional, a policymaker, or if you just want to know more about biosimilars, the Biosimilars Patient Resource Center has information you can use.
Additional Resources
Filter by Keyword
More results...
- All
- Backgrounder
- Biosimilars Bulletin
- Blog
- Council Resource
- Event
- Handbook
- Infographic
- Issue Brief
- Member Spotlight
- Partner Resource
- Policy Recommendation
- Press Release
- Report
- Streamlining
- Video
- White Papers

September 25, 2024
Blog

December 18, 2023
Report

July 18, 2023
Biosimilars Bulletin

September 21, 2022
Report

October 12, 2021
Report

April 23, 2021
Press Release

September 28, 2020
White Papers

October 24, 2019
Member Spotlight

May 13, 2019
Council Resource
White Papers
September 25, 2018
Blog

September 12, 2018
Event

August 28, 2018
Partner Resource

August 24, 2018
Partner Resource
August 18, 2018
Partner Resource